April 1, 2021 at 10:23 pm | Updated April 1, 2021 at 10:23 pm | 6 min read
Improving the vase life and quality of flowers is vital for growers, as cut flowers have a very short postharvest life and customer treatment of the flowers once they are cut is beyond the growers’ control. However, to protect their brand name, growers can still ensure a longer flower life and better quality by using preharvest sprays. Scientists Kaviya, S. S., Lourdusamy, K., Ganga, M., & Vincent, S., used precise gas analysis instruments to fine-tune these sprays and produce a better harvest.
Factors Affecting Cut Flowers’ Vase Life
It is quite common to see flowers in the supermarket that have been imported from other countries. After being cut, the flowers must remain fresh throughout transport, storage at retailers and then remain fresh in consumer vases. However, 30-70% of the flower quality depends on farm conditions. There are many preharvest factors during flower farming that reduce flower quality and vase life:
- Water stress: Water deficiency during flower development will impact their vase life later. Moreover, the plants should be watered well prior to cutting to ensure that the cells in the flowers and stem are turgid.
- Infection by microorganisms: Infection due to soil splash on plants will affect the longevity of flowers.
- Ethylene production in plants: Ethylene is a phytohormone released by the plants and is necessary for germination, sprouting, root development, stem elongation, and ripening. It also causes senescence of flowers and leaves. Several flowers are sensitive to ethylene and even trace amounts of twenty parts per billion can cause wilting of flowers. Besides the death of flowers, the gas also impacts bud development and induces abscission.
The Peruvian Lily, or Alstroemeria, is no exception. Exported internationally, this flower is popular for its large flowers and vibrant colors; see Figure 1.
Subscribe to the Felix instruments Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact

Figure 1: Image by Meatle and bassoon12345 from Pixabay
The leaves on the Peruvian Lily turn yellow and age within a few days, and although the life of the flowers themselves is long, the petals will fall off.
In other lily varieties, as well as in the rose, growers solve this problem by using many sprays to keep leaves green to preserve the appearance of the cut flowers and extend vase life. Some of the popular pre-harvest sprays are Salicylic acid, Gibberellic acid, and Benzyl Adenine.
Testing Pre-Harvest Sprays
A group of Indian scientists, Kaviya et al. decided to test the effect of these pre-harvest sprays on the leaf and flower of the Peruvian Lily (Alstroemeria hybrida cv. Pink Panther).
Besides a control treatment, they used three concentrations of the pre-harvest sprays:
- Salicylic acid- 50, 100 & 150 µM
- Benzyl Adenine (BA) -50, 100 & 150 µM
- Gibberellic acid (GA3) -200, 250 & 300 ppm
The sprays were applied two weeks and one week before harvest during flower farming. The flowers were cut during the budding stage. The flower stems were immediately cooled, graded, packed, and sent to the laboratory. There, following standard industry practice, the stems were cut underwater and kept in a vase of 100 ml distilled water.
Data was collected once every two days—on the 2nd, 4th, 6th, and 8th day—until the end of vase life. The vase life was taken as the number of days when 50% of florets wilted and 50% of leaves were yellow. The following parameters were recorded:
- The relative weight of flowers and the vase solution uptake
- Floret diameter
- Chlorophyll content, which was measured by extracting it from 250 mg of leaves with acetone and measuring the optical density of the supernatants with a UV spectrophotometer.
Ethylene levels in the flowers were measured continuously. So, the scientists needed a non-destructive gas analysis instrument that was precise and easy to use. The scientists measured ethylene production at 9 am and 9 pm on all eight days. They used the F-950 Three Gas Analyzer by Felix Instruments – Applied Food Science. The device was sensitive enough to measure minute levels of gas. It has a lower detection limit of 0.15 ppm and a resolution of 0.1 ppm. Also, the results were obtained rapidly in 35 seconds for each reading.
The F-950 is a small portable gas analyzer that measures ethylene, as well as carbon dioxide and oxygen.
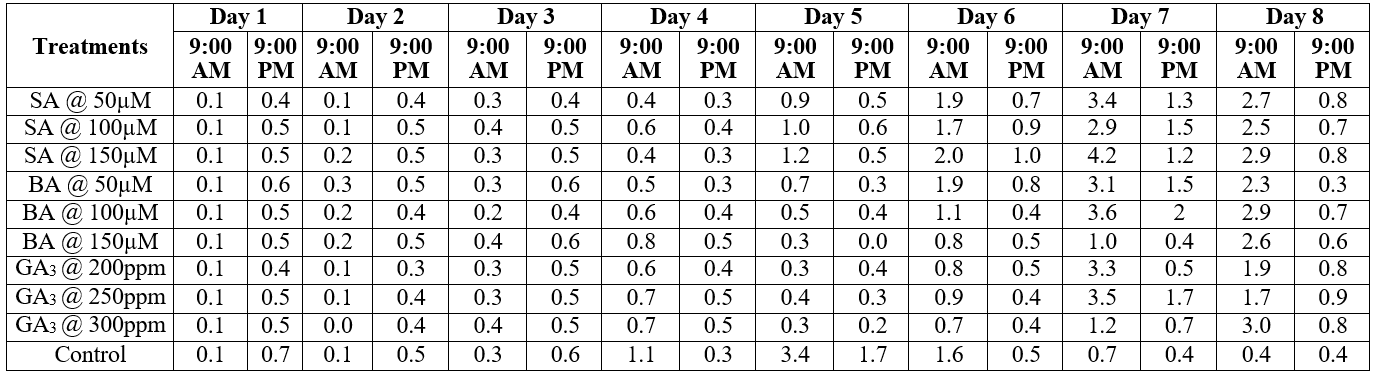
Table 1: “Effect of various treatments on ethylene production (in ppm) of Alstroemeria flowers,” Kaviya, et al. 2021. (Credits: Journal of Pharmacognosy and Phytochemistry, 10(1): 429-433. DOI: 10.22271/phyto.2021.v10.i1Sg.13597)
The device uses an electrochemical cell to record ethylene production. The scientists connected the probes to the intake of the F-950, and connected a needle filter to the tube to prevent moisture from entering the gas analysis instrument. They then attached a sterile needle to the filter. This sterile needle was injected into the sealed polythene bags containing the flower stems. Ethylene production was recorded in parts per million (ppm) for all the treatments, as shown in Table 1.
Preharvest Sprays of Benzyl Adenine and Gibberellic Acid
The relative weights of flowers increased in the first four days across all treatments due to uptake of vase solution. After the fourth day, the fresh weight decreased due to lower uptake.
Treatment with 300 ppm of GA3 (88.5%) produced the highest relative fresh weight, followed by 150 µM of Benzyl Adenine (87.18%), while the control gave the lowest weight (51.19%).
The largest florets were also produced by the 300 ppm of GA3 and the least amount of florets were produced by controls. Better absorption of the solution increased cell division and accumulation of nutrients and water by the flowers.
As days went by, the chlorophyll level steadily decreased in all treatments. On the eighth day, the highest chlorophyll content was seen in flowers from plants treated with 300 ppm of GA3. The next best treatment was, again, by 150 µM of Benzyl Adenine, and the control performed the worst.
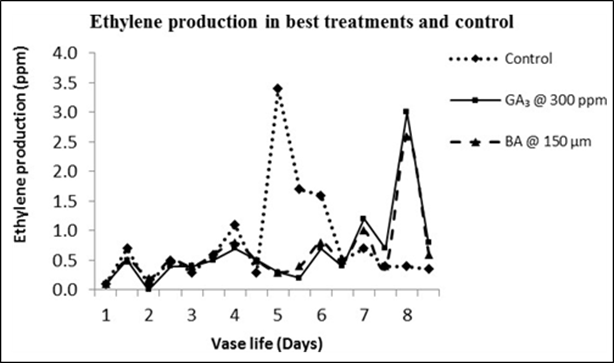
Fig 1: “Ethylene evolution in control flowers, flowers treated with GA3 @ 300 ppm and BA @ 150 µM,” Kaviya, et al. 2021. (Credits: Journal of Pharmacognosy and Phytochemistry, 10(1): 429-433. DOI: 10.22271/phyto.2021.v10.i1Sg.13597)
Ethylene did not have much effect on leaf senescence, and yellow leaves were recorded even at very low ethylene production levels. For flowers, ethylene production was more important only at the later stages of vase life, and it did not reduce flower life.
Gibberellic acid and Benzyl Adenine were successful in dampening ethylene production. In controls, ethylene production peaked on the 5th day, whereas the Gibberellic acid and Benzyl Adenine treatments saw spikes only on the last day; see Figure 2 for the gas analysis trends.
The Benzyl Adenine treatment of 150 µM produced the longest living flowers, lasting for eight days. The control flowers wilted the earliest – after three days.
The scientists concluded that the salicylic acid treatment was not beneficial in any way. Though Benzyl Adenine @ 150 µM increased postharvest life, it was Gibberellic acid that enhanced the quality of flower stem by producing the largest flowers and keeping leaves green. Since GA3 is also the cheapest and most easily available chemical, the researchers recommend its use as a preharvest spray.
Best Practices for a Long Vase Life
Flower growers put in a lot of effort to keep flowers fresh. However, it is not just during the preharvest stage where they can influence vase life. The University of Massachusetts Extension Greenhouse Crops and Floriculture Program suggests some of the following harvest and handling tips, which can optimize vase life and flower quality:
Timing of harvest: Cut flowers in the morning or evening and not in the afternoon.
Avoid contamination risk: Hygiene is important while cutting and handling the flowers at harvest to prevent microbial infection that can spoil flowers. Some best practices are to
- disinfect tools,
- use clean buckets and clean water to store flowers after cutting,
- keep work surface clean,
- and not place flowers on the floor.
Provide nutrition: Provide sugars, as flowers continue to respire after cutting and use their stored sugar content.
Avoid overcrowding: Do not put too many flowers in a single bucket.
Operating in the shade: Perform all operations in the shade to lower temperatures and maintain flower freshness.
Harvest in small batches: Cut the flowers in small batches so that they can be cleaned, graded, and moved quickly into a cool store, without a long time gap, to preserve freshness.
Post-harvest care: After moving flowers to a cool place, recut stems underwater to prevent air bubbles from entering the stems and place them in solutions. These are for hydrating, holding, and anti-ethylene solutions. Organic flower suppliers cannot use many of the chemical formulations, so they have to be extra vigilant about maintaining proper sanitation during and after harvest.
Store upright: Flower stems are geotropic and grow away from gravity, so they should be stored in an upright position. Storing them horizontally will make the flowers bend away from the surface.
Improving Flower Farming
Flower farming is a lucrative multibillion-dollar business; it was worth US$29.2 Billion in 2020. Cut flowers are the most delicate among farm products and growers can use every ounce of help they can get to keep the flowers healthy and looking good. Simple cost-effective measures like preharvest sprays are a boon for their business. By preventing waste, the growers make agriculture more sustainable and profitable.
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature photo courtesy of Abspires40
Sources
Kaviya, S. S., Lourdusamy, K., Ganga, M., & Vincent, S. (2021). Influence of pre-harvest sprays on flower quality and vase life of Alstroemeria. Journal of Pharmacognosy and Phytochemistry, 10(1): 429-433. DOI: 10.22271/phyto.2021.v10.i1Sg.13597
ReportLinker. (2020, August 24). Global cut Flowers industry. Retrieved from https://www.globenewswire.com/news-release/2020/08/24/2082757/0/en/Global-Cut-Flowers-Industry
University of Massachusetts Amherst. (2016). Harvesting and Handling Cut Flowers. Retrieved from https://ag.umass.edu/greenhouse-floriculture/fact-sheets/harvesting-handling-cut-flowers
Related Products
- F-751 Grape Quality Meter
- Custom Model Building
- F-901 AccuRipe & AccuStore
- F-751 Melon Quality Meter
- F-751 Kiwifruit Quality Meter
- F-750 Produce Quality Meter
- F-751 Avocado Quality Meter
- F-751 Mango Quality Meter
- F-900 Portable Ethylene Analyzer
- F-950 Three Gas Analyzer
- F-920 Check It! Gas Analyzer
- F-960 Ripen It! Gas Analyzer
- F-940 Store It! Gas Analyzer
Most Popular Articles
- Spectrophotometry in 2023
- The Importance of Food Quality Testing
- NIR Applications in Agriculture – Everything…
- The 5 Most Important Parameters in Produce Quality Control
- Melon Fruit: Quality, Production & Physiology
- Guide to Fresh Fruit Quality Control
- Liquid Spectrophotometry & Food Industry Applications
- Ethylene (C2H4) – Ripening, Crops & Agriculture
- Fruit Respiration Impact on Fruit Quality
- Active Packaging: What it is and why it’s important






