February 4, 2021 at 11:46 pm | Updated February 4, 2021 at 11:46 pm | 7 min read
Ethylene produced by many fungi can cause disease in perishable fresh produce. Scientists found that this phytohormone can be used as a marker in non-destructive diagnosis for fruit disease detection of post-harvest fruits. A portable gas analyzer was used in an experiment to measure fungal ethylene in real-time. The findings from the experiment could help in reducing food loss.
Methods of Fungal Fruit Disease Detection
Around 40-50% of fresh produce is lost in the post-harvest stage due to rotting. Fungi alone can account for the destruction of 22.5% of fruits and vegetables.
In tomatoes and grapes, the high water content and thin skin make them very vulnerable to fungal attacks during extended storage. Bruising, transport and harvest damage, presence of fungi before harvest, or improper storage conditions encourage the growth and spread of fungi in storerooms. Certain pathogens can produce mycotoxins that are harmful to people, so they are also a safety risk.
Subscribe to the Felix instruments Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Being able to detect fungal presence and damage early can limit the spread of diseases. Traditional fruit disease detection methods rely on microscopic examination, isolation, and culture to find pathogens. These methods are destructive, laborious, and time consuming. Being restricted to spot sampling, they introduce estimation error in disease burden.
Hence, scientists have been trying to use physiological indices based on markers to find a better fruit disease detection method. Ethylene production had been noticed during disease occurrence, but researchers wanted to be sure of the cause, since stress can also trigger the production of this phytohormone.
A group of Chinese scientists—Guo, Liu, Y. Wang, T.Wang, Zhang, Zhu, and Xu—knew that ethylene production in plants infected by fungus was often greater in high-light vs low-light conditions. However, they were not sure to what degree the presence and absence of light influenced ethylene production in diseased plants.
In their experiment, the scientists tested the ethylene production of plant material infected by the fungus Botrytis cinerea (strain B05.10), expressing the GFP marker in both darkness and light.
The fresh produce chosen for these tests were tomato (Solanum lycopersicum, the cherry tomato cultivar) and grapes (Vitis vinifera, the Shine-Muscat cultivar).
Challenge: Finding a Non-destructive Mode of Ethylene Measurement
The scientists conducted four different experiments to examine various aspects:
- Besides B. cinerea, Colletotrichum gloeosporioides, Penicillium digitatum, Alternaria alternata, and Fusarium asiaticum fungi were cultured and the pelleted spores were mixed in water to check if they produced ethylene.
- To test for the effect of light, invivo tests were created. Spores were added to a medium and grown in a petri dish for two days, after which they were subjected to two light treatments: dark and light from LEDs.
- Arabidopsis thaliana seedlings were used to check for ethylene production as a result of B. cinerea fungal infection in plants, with uninfected plants as controls. After four days, plants were again divided and kept in both dark and light conditions.
- Tomato and grape fruits were purchased from the market, and wounded, freeze treated, or injected with B. cinerea spores. After twenty-four hours, they were also kept in light and dark conditions for one hour and tested for ethylene in real-time.
In the last three experiments, ethylene measurements were taken from samples kept in the light. Control samples kept in the dark were moved into the light for a short time.
In all experiments, the fungal ethylene production was measured by gas chromatography and had to be measured in real-time by a non-destructive method.
For the non-destructive measurements, the scientists needed a portable gas analyzer that was accurate, could measure low levels of ethylene, and was effective in a wide range of temperature and humidity conditions.
There are several portable gas analyzers on the market. Micro gas chromatography systems are available, but a preconcentration step is necessary to improve sensitivity, so getting real-time readings is not possible. Optical gas detectors can give readings in real-time, but the accuracy depends on the price of the tool and sensors used.
Solution: Felix F-950 Three Gas Analyzer
The scientists decided to use an electrochemical sensor-based tool, the Felix F-950 Three Gas Analyzer. This gas analysis instrument has been designed to operate in a wide range of temperature and humidity conditions, unlike other electrochemical sensors, which can operate only within a narrow range of environmental conditions.
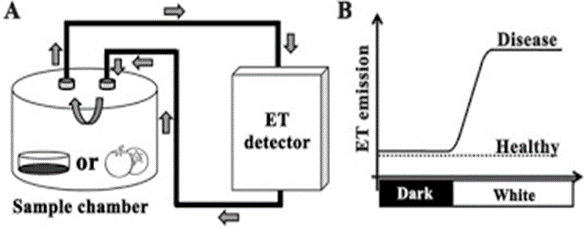
Figure 1 A. Diagram showing the experimental setup for real-time measurement with F-950. “Petri dishes containing fungal cultures in agar medium (left) or infected fruits (right) were placed in the sealed sample chamber, which was connected to the gas analyzer by airtight rubber tubes. Arrows in the figure represent airflow direction during measurement. B, the model for measuring fungal ET induced by the DTL transition to predict the risk of disease in harvested fruit crops,” Guo, et al. 2020. (Image credits: https://doi.org/10.1016/j.foodchem.2019.125827)
To measure fungal ethylene production in real-time, as shown in Figure 1 A, the Petri dishes or fruit samples were kept in an airtight chamber and connected to the inlet and outlet of the portable gas analyzer F-950 with air-tight needles and tubes. A micropump created a circulating airflow, and ethylene levels were measured every second.
Benefits of Using the Portable Gas Analyzer
The scientists had selected the F-950 due to its ability to measure ethylene levels ranging from 0.1 to 100 parts per million (ppm). Thus, they were certain they would get accurate results.
Use of the instrument with a wide variety of fruits had already established, and the small, portable form factor made it a convenient tool for the researchers. Moreover, scientists needed only 30 seconds to record each reading, so the requirement of getting real-time measurements was also fulfilled.
A battery with a charge lasting for eight hours allowed data collection of the various samples without interruption.
A 16 GB SD card stored the data logged by the portable gas analyzer for statistical analysis without the hassle of data entry, saving them considerable time. This data was easily transferred to the scientists’ computers for further use.
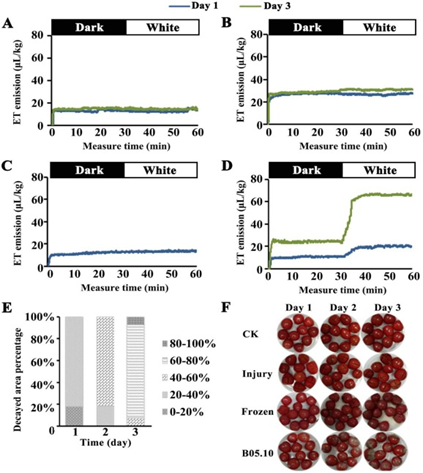
Figure 2: “Measurement of the ET accumulation of the tomato-Botrytis pathosystem in response to the DTL transition. A-D, ET accumulation of the healthy (A), physically injured (B), freeze/thawed (C), and Botrytis-infected (D) tomatoes, as measured continuously for 30 min in the dark, followed by 30 min in white light. E, graphical representation of decay development of tomatoes infected with B. cinerea. F, phenotypes of each group of fruit samples. CK, control group for healthy fruits; Injury, physically injured; Frozen, treated with freezing at −20 °C for 2 h; B05.10, infected with B. cinerea,” Guo, et al. 2020. (Image credits: https://doi.org/10.1016/j.foodchem.2019.125827)
Portable Gas Analyzer Shows Fungal Ethylene Can Be a Marker
Using real-time measurements by the gas analysis instrument, the scientists were able to show that all five species of fungi produced ethylene in Petri dishes, and all of them were affected by the transition from dark to light. Besides white light, blue and red-colored light provided by LEDs were also tested. Blue light triggered the maximum production of fungal ethylene in the first few minutes light exposure.
This trend was confirmed in fungi and plant interaction. A. thaliana seedlings showed increased fungal ethylene when infected with fungi and under light. Even the addition of an ethylene inhibitor, PZA, could not lower the fungal ethylene levels in infected seedlings.
Figure 2 shows that healthy climacteric tomatoes, only physically wounded or frozen, have the same ethylene levels in dark and light, while tomatoes infected with B. cinerea show more fungal ethylene the longer they are infected and after the transition to light from darkness.
Non-climacteric grapes, which do not produce ethylene for ripening, also registered higher levels of fungal ethylene in infected fruits and after exposure to light. Mycelial growth was confirmed by microscopic examination of the berries, as shown in Figure 3. On day two, we see fungal ethylene production and the presence of mycelium inside the berry, even though the fruit looks healthy on the exterior.
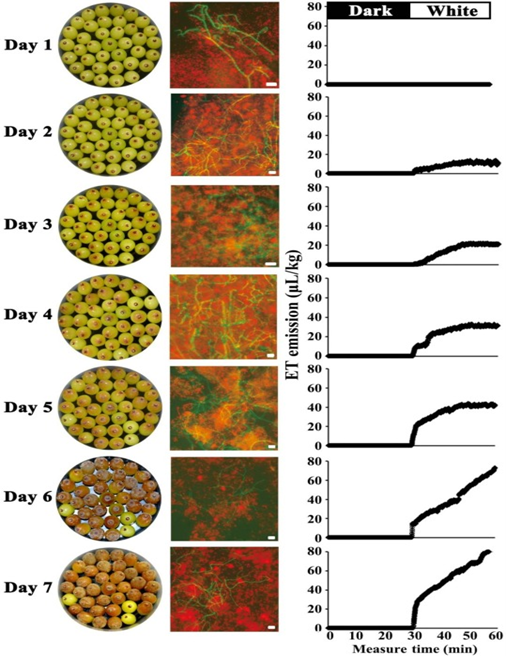
Figure 3: “Evolution of ET accumulation in response to the DTL transition in the grape-Botrytis pathosystem. The B. cinerea strain carrying a GFP-expressing vector was inoculated onto grape berries. Symptom development, including grey mold and decay, was recorded by photos (left lane pictures), fungal hyphal growth was observed by fluorescence microscopy with free-hand section samples of the infected grape tissues (middle lane pictures). The ET accumulation of the pathosystem in response to the DTL transition, as measured by the real-time system (as described in Fig. 1), and the result is shown in the right lane,” Guo, et al. (Image credits: https://doi.org/10.1016/j.foodchem.2019.125827)
The scientists recommend the testing chamber setup described in Figure 1 for fruit disease detection. If the levels of ethylene remain the same in dark and after the transition to light, the fruits are healthy. A rise in ethylene gas levels after a transition from dark to light should be considered a marker of fruit disease, regardless of external appearance.
Immediate Benefits to the Supply Chain
Early, non-destructive fruit disease detection, even before external symptoms develop, has several advantages. Suppliers and distributors can cull contaminated batches and limit the spread of diseases to reduce yield losses. They can also sort the produce efficiently and select clean batches for long-distance transport or extended storage. The retailer is assured a good price and the consumer assured healthy food. Since the F-950 was designed for industrial use, these research findings have immediate practical applications. The scientists identified not only a marker, but also a readily available tool that can be easily used throughout the supply chain for checking fresh produce.
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of Scot Nelson.
Sources
Alkan, N., & Fortes, A. N. (2015). Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens.Front. Plant Sci.,6:889. DOI=10.3389/fpls.2015.00889
Guo, H., Liu, A., Wang, Y., Wang, T., Zhang, W., Zhu, P., & Xu, L. (2020). Measuring light-induced fungal ethylene production enables non-destructive diagnosis of disease occurrence in harvested fruits. Food Chemistry 310. DOI: https://doi.org/10.1016/j.foodchem.2019.125827
Pétriacq, P., López, A., & Luna, E. (2018). Fruit Decay to Diseases: Can Induced Resistance and Priming Help? Plants. 7(4):77. https://doi.org/10.3390/plants7040077
Related Products
- F-751 Grape Quality Meter
- Custom Model Building
- F-901 AccuRipe & AccuStore
- F-751 Melon Quality Meter
- F-751 Kiwifruit Quality Meter
- F-750 Produce Quality Meter
- F-751 Avocado Quality Meter
- F-751 Mango Quality Meter
- F-900 Portable Ethylene Analyzer
- F-950 Three Gas Analyzer
- F-920 Check It! Gas Analyzer
- F-960 Ripen It! Gas Analyzer
- F-940 Store It! Gas Analyzer
Most Popular Articles
- Spectrophotometry in 2023
- The Importance of Food Quality Testing
- NIR Applications in Agriculture – Everything…
- The 5 Most Important Parameters in Produce Quality Control
- Melon Fruit: Quality, Production & Physiology
- Guide to Fresh Fruit Quality Control
- Liquid Spectrophotometry & Food Industry Applications
- Ethylene (C2H4) – Ripening, Crops & Agriculture
- Fruit Respiration Impact on Fruit Quality
- Active Packaging: What it is and why it’s important






