September 15, 2025 at 4:21 pm | Updated September 15, 2025 at 4:21 pm | 8 min read
- Ethylene must be monitored and controlled as even trace amounts in parts per billion can cause over-ripening, decay, and spoilage.
- Fixed and portable gas analyzers that can detect and monitor ethylene levels in storage facilities are available on the market.
- Several strategies exist to lower ethylene levels, including venting, the use of ethylene inhibitors, scrubbers, absorbers, and scavengers.
To preserve sensory and internal quality as well as the nutritional value and safety of fresh produce, ethylene concentrations must be monitored and controlled in the postharvest supply chain. Ethylene from both exogenous and endogenous sources must be eliminated in the process. This article presents a brief overview of ethylene detection and control, as an introduction to the detailed and product-specific information that exists in the industry.
Why Ethylene Needs to Be Controlled
Ethylene is a valuable chemical widely used in the fresh produce industry; however, it can also have adverse effects and is responsible for a significant proportion of fresh produce losses in the food industry.
Ethylene is a phytohormone that all plants produce in various stages of their lifecycle. It is essential for germination, sprouting, root development, stem elongation, leaf and flower abscission, and ripening of climacteric fruits. It is also the hormone that initiates senescence and acts as a stress response. Non-climacteric fruits do not need ethylene for ripening, but some are sensitive to its other effects, as shown in Table 1.
Subscribe to the Felix instruments Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
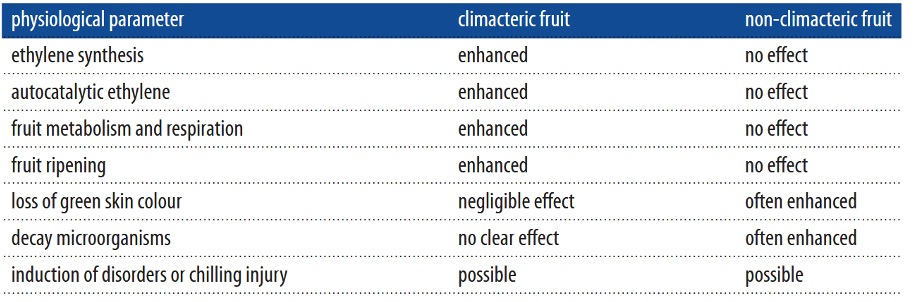
Table 1. “Physiological effects of exogenous ethylene on fruit and vegetables as dependent on their climacteric classification,” Janssen et al. 2014. (Credits: https://pmc.ncbi.nlm.nih.gov/articles/PMC4006173/pdf/rsta20130311.pdf)
The adverse effects of ethylene occur postharvest when fruits are exposed unintentionally to ethylene and trigger the following processes:
- Unplanned ripening in climacteric fruits
- Senescence in fresh produce
- Makes fresh produce more susceptible to physiological disorders and pathogens
These effects depend on the sensitivity of fresh produce to ethylene. In addition, ethylene also increases the respiration rate in fruits. Species, cultivar, ethylene concentration, exposure duration, temperature, and humidity in the postharvest supply chain can influence the extent of damage ethylene causes.
Ethylene concentrations from one part per billion (ppb) can reduce the quality and shelf-life of fresh produce. Ethylene’s devastating effects occur because the chemical is very reactive and is involved in many crucial postharvest physiological processes.
Ethylene effects can be stopped in non-climacteric fruits if the external source is removed, since they do not themselves produce the phytohormone. However, once climacteric fruits are exposed to exogenous ethylene, it triggers internal ethylene synthesis, resulting in high losses in these fruits.
Stakeholders in the fresh produce supply chain try to control its effects by preventing endogenous biosynthesis or absorption from exogenous sources. If ethylene is not removed, it causes over-ripeness, premature decay, and microbial spoilage that can result in unpleasant odors, discoloration, bitterness, and loss of weight and color.
Detecting and Monitoring Ethylene
Climacteric fruits are themselves a significant source of ethylene in the food supply chain, as the gas spreads rapidly in the air. Moreover, residual ethylene in compartments where climacteric fruits were previously stored can also affect future batches of fruits. For example, in Australia, an empty storage that had previously held avocados had 280 ppb of ethylene.
Exogenous sources of ethylene that can affect non-climacteric and climacteric fruits are present in the postharvest environment. These can be produced from the burning of fossil fuels, stubble, smoke, or adjacent crops.
Preventing ethylene effects first requires that the air be monitored to find the levels of the gas present. Closed rooms and containers with less ventilation often result in a buildup of the gas when it cannot escape.
Even a decade ago, ethylene monitoring was not widely prevalent, according to Janssen et al. (2014). Controlled atmosphere (CA) units are routinely checked for oxygen and carbon dioxide gases. Still, ethylene levels were estimated only on a daily or weekly basis, due to less access to reliable measuring instruments. Ethylene estimation was primarily done in laboratories.
So, ethylene estimation at points of origin is often not optimal. Moreover, many instruments are sensitive to the high humidity that is maintained in storage and transport facilities, further complicating ethylene measurement.
Ethylene can be detected through portable and fixed sensors. The standard technology used is electrochemical sensors, which are capable of measuring the gas at ppb levels necessary for climacteric fruits.
Felix Instruments offers industry-standard portable and fixed sensors suitable for use in many points in the fresh produce supply chain for storage and headspace and flux analysis of samples with trace ethylene rates. These instruments are precise, easy to use, and estimate ethylene levels in real time:
- F-901D AccuStore measures ethylene from 0-5 ppm. It has a controller to manage the levels of gas. The instruments also measure temperature and humidity, which affect ethylene estimation. The instrument is fixed inside a room and can be operated remotely by staff.
The portable devices offered by Felix Instruments are as follows:
- F-900 Portable Ethylene Analyzer estimates the gas in the range of 0-10 ppm and is precise enough for use in the food industry and research for ripening gases.
- F-940 Store It! A gas analyzer can measure the concentrations of three gases —ethylene, carbon dioxide (CO2), and oxygen (O2) —simultaneously in CA and ripening rooms, as well as in modified atmosphere packages.
- F-950 Three Gas Analyzer measures ethylene from 0-200 ppm along with the gases CO2 and O2.
- F-960 Ripen It! Gas Analyzer measures ethylene in the 10-1000 ppm range.
Technologies to Control Ethylene Levels
Various technologies have been developed over the past two decades to control and reduce ethylene levels during storage and transport, thereby extending the shelf life of perishables. These technologies are discussed below.
-
Housekeeping
The simplest method to reduce ethylene is good housekeeping.
- Storing separately: There is ample information on ethylene producers and the species sensitive to the phytohormone, and the optimal storing conditions for extending storage duration. See Table 2 for a few examples. The ethylene-sensitive species should not be kept in the same room as crops that produce ethylene or where exogenous sources exist.
- Cull overripe fruits: Damaged, spoiled, and rotting fruits that are ethylene-producers should be removed from storage.
2. Cooling
Fresh produce is precooled and transferred as soon as possible into cold storage to control respiration and ethylene synthesis. Higher temperatures and increased respiration rates can both increase ethylene production.
Table 2: “Storage Recommendations and Ethylene Sensitivity of Some Perishables, Wray-French, L. (2015). (Credits: DOI:10.13140/RG.2.1.4477.5848)
| Common Name | Recommended Storage Temperature oC | Ethylene Production* | Ethylene Sensitivity** | Approximate Storage Life |
| Apple | -1.1 to 0 | VH | H | 3-6 months |
| Banana | 13 to 15 | M | H | 1-4 weeks |
| Broccoli | 0 | VL | H | 10-14 days |
| Carrot | 0 | VL | H | 3-6 months |
| Cauliflower | 0 | VL | H | 3-4 weeks |
| Cherry | -1 to 0 | VL | L | 2-3 weeks |
| Cucumber | 10 to 12 | L | H | 10-14 days |
| Grapefruit | 10 to 15 | VL | M | 6-8 weeks |
| Kiwifruit | 0 | L | H | 3-5 months |
| Lettuce | 0 | VL | H | 2-3 weeks |
| Mango | 13 | M | M | 2-3 weeks |
| Melon, cantaloupe | 2 to 5 | H | M | 2-3 weeks |
| , honey-dew | 5 to 10 | M | H | 3-4 weeks |
| Mushroom | 0 | VL | M | 7-14 days |
| Nectarine | -0.5 to 0 | M | M | 2-4 weeks |
| Orange | 3 to 9 | VL | M | 3-8 weeks |
| Peach | -0.5 to 0 | M | M | 2-4 weeks |
| Pear | -1.5 to -0.5 | H | H | 2-7 months |
| Strawberry | 0 | L | L | 7-10 days |
| Tomato, green | 10 to 13 | VL | H | 2-5 weeks |
*Ethylene production rate: **Ethylene sensitivity
VL = very low (<0.1µL/kg-hr at 20oC) L = low sensitivity
L = low (0.1µL/kg-hr) M = moderately sensitive
M = moderate (1.0-10.0µL/kg-hr) H = highly sensitive
H = high (10-100µL/kg-hr)
Source: Adapted from Cantwell, M, UC Davis 2001.
-
Venting
Ventilation and proper air circulation are maintained to prevent the possibility of ethylene gas accumulating in areas where ethylene-sensitive and climacteric fruits are stored, thereby preventing decay and unplanned ripening. Fresh ambient air that has only 1–6 ppb ethylene is introduced on long-haul ships.
-
Inhibitor of ethylene receptors
Ethylene inhibitors are also placed in containers and rooms to reduce ethylene perception. Inhibitors are substances that block ethylene receptors in fresh produce and hinder processes such as ethylene biosynthesis, perception, or signaling. Reducing and slowing down these processes restricts ripening, as shown in Figure 1. The most common ethylene inhibitors are
- 1-Methylcyclopropene (1-MCP)
- Salicylic acid (SA),
- Aminoethoxyvinylglycine (AVG)
- Amino-oxyacetic acid (AOA)
Figure 1: The pathways through which receptors and scavengers control ethylene levels, Ebrahimi et al. (2021). (Image credits: https://doi.org/10.1007/s13762-021-03485-X)
-
Ethylene scavengers
Ethylene scavengers remove the gas by catalytic oxidation, where ethylene (C2H4) is broken down irreversibly into carbon dioxide and water, see Figure 1. The oxidizing capacity of scavengers determines how efficiently ethylene is removed. The common substances and technologies used as scavengers are as follows:
- Potassium permanganate (KMnO4)
- Ozone (O3)
- Palladium
- Titanium dioxide (TiO2)
- UV radiation
- Cold plasma
-
Ethylene absorbers and scrubbers
Ethylene absorbers trap the gas through absorption and adsorption, where ethylene molecules are held inside and on the surface of the solid, respectively. See Figure 2, which shows ethylene gas molecules absorbed by activated carbon. The solids are placed in sachets and used in CA rooms or modified atmosphere packaging (MAP). The standard scrubbers are:
- Zeolite
- Activated carbon
- Metal–organic frameworks
- Silica gel
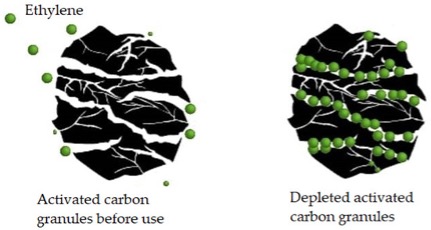
Figure 2. “Active carbon acts as an ethylene adsorbent. When this phytohormone penetrates through the structural cracks in the active carbon, it remains adhered to the walls, thus preventing its effect,” Alonso-Salinas et al. 2024. (Image credits: https://www.mdpi.com/2311-7524/10/8/840)
One or more of these technologies can be applied during storage and transport to control ethylene in commercial setups.
Industrial Applications
The fresh produce industry uses controlled atmosphere storage (CA) and modified atmosphere packing (MAP) to extend shelf-life, especially in long-distance supply chains. Ethylene monitoring and control should be an integral part of these operations to curb losses.
Controlled Atmosphere Storage
CA storage involves manipulating the atmosphere, where harmful gases are reduced and the level of beneficial gases is increased. Low temperatures and high relative humidity are also maintained in these facilities. The gas concentrations are altered so that higher-than-normal CO2 and lower-than-ambient levels of O2 are used to reduce respiration and ethylene production rates. Monitoring and controlling the levels of all three gases, ethylene, CO2, and O2, is essential. Ethylene inhibitors, scrubbers, scavengers, venting, or housekeeping are used to lower ethylene levels. CA is expensive and reserved for long-term storage of apples, pears, kiwis, and cabbage.
Modified Atmosphere Packaging
MA also alters the levels of the three gases in individual packages of whole and cut fresh produce. Sometimes, inert gases like nitrogen can also be added to the headspace. The packaging material or sachets of ethylene scavengers are used to lower ethylene levels in packages to extend storage time.
Integrating Instruments
Instruments that can monitor ethylene and control the gas levels should be an integral part of fresh produce storage, given the potential damage and losses that the gas can cause in the post-harvest stages. Reliable instruments that estimate ethylene levels in real time, onsite, and support services can improve yield and ROI for stakeholders.
Contact us for more information on Felix Instruments Applied Food Science products for ethylene monitoring and control.
Sources
Alonso-Salinas, R., López-Miranda, S., Pérez-López, A. J., & Acosta-Motos, J. R. (2024). Strategies to delay ethylene-mediated ripening in climacteric fruits: Implications for shelf life extension and postharvest quality. Horticulturae, 10(8), 840.
Asrey, R., Sharma, S., Barman, K., Prajapati, U., Negi, N., & Meena, N. K. (2023). Biological and postharvest interventions to manage the ethylene in fruit: a review. Sustainable Food Technology, 1(6), 803-826.
Blanke, M. Challenges of Reducing Fresh Produce Waste in Europe—From Farm to Fork. Agriculture, 5(3), 389-399. https://doi.org/10.3390/agriculture5030389
Caprioli, F., & Quercia, L. (2014). Ethylene detection methods in post-harvest technology: A review. Sensors and Actuators B: Chemical, 203, 187-196.
Ebrahimi, A., Zabihzadeh Khajavi, M., Ahmadi, S., Mortazavian, A. M., Abdolshahi, A., Rafiee, S., & Farhoodi, M. (2022). Novel strategies to control ethylene in fruit and vegetables for extending their shelf life: A review. International Journal of Environmental Science and Technology, 19(5), 4599-4610.
Janssen S, Schmitt K, Blanke M, Bauersfeld ML, Wöllenstein J, Lang W. 2014. Ethylene detection in fruit supply chains. Phil. Trans. R. Soc. A 372: 20130311.
http://dx.doi.org/10.1098/rsta.2013.0311
Wray-French, L. (2013). A review of ethylene management techniques controlling the shelf life of perishables, and research into two new types of ethylene scrubbing technologies. Food Safety and Quality Management Masters.
Related Products
- F-751 Grape Quality Meter
- Custom Model Building
- F-910 AccuStore
- F-751 Melon Quality Meter
- F-751 Kiwifruit Quality Meter
- F-750 Produce Quality Meter
- F-751 Avocado Quality Meter
- F-751 Mango Quality Meter
- F-900 Portable Ethylene Analyzer
- F-950 Three Gas Analyzer
- F-920 Check It! Gas Analyzer
- F-960 Ripen It! Gas Analyzer
- F-940 Store It! Gas Analyzer
Most Popular Articles
- Spectrophotometry in 2023
- The Importance of Food Quality Testing
- NIR Applications in Agriculture – Everything…
- The 5 Most Important Parameters in Produce Quality Control
- Melon Fruit: Quality, Production & Physiology
- Fruit Respiration Impact on Fruit Quality
- Guide to Fresh Fruit Quality Control
- Liquid Spectrophotometry & Food Industry Applications
- Ethylene (C2H4) – Ripening, Crops & Agriculture
- Active Packaging: What it is and why it’s important






